Nylon6 vs Nylon66

Nylon 6 and nylon 66 are both types of nylon, a strong and versatile plastic widely used for fabrics, engineering parts, and more.
History:
- Nylon was first invented in the 1930s by Wallace Carothers at DuPont.
- Nylon 66 was the first commercially successful nylon, introduced in 1940.
- Following this in 1939, nylon 6 as it was to be known, was created in Germany by Paul Schlack, developed as a simpler and more cost-effective alternative.
Raw Materials:
-
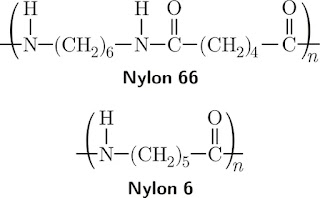
Nylon 6: Made from caprolactam, a ring-shaped molecule with 6 carbon atoms.
-
Nylon 66: Made from two different molecules - adipic acid and hexamethylenediamine, both with 6 carbon atoms each.
Manufacturing Process:
Both nylons involve a similar process:
- Polymerization: The raw materials undergo a chemical reaction to form longchains of molecules (polymers).
- Melting: The polymers are melted into a liquid form.
- Shaping: The molten nylon is then extruded (pushed through a mold) or spun into fibers for fabrics.
- Solidification: The nylon cools and hardens into its final form.
Properties Comparison
| Property | Nylon 6 | Nylon 66 |
|---|---|---|
| Chemical Structure | Polycaprolactam (single 6-carbon monomer) | Hexamethylene diamine & Adipic Acid (two 6-carbon monomers) |
| Melting Point (ºC) | 215 | 265 |
| Softening Point (ºC) | 175 | 240 |
| Tensile Strength (MPa) | 70-80 | 75-90 |
| Elastic Modulus (GPa) | 2.4-3.0 | 3.0-4.0 |
| Impact Strength (Izod notched, kJ/m) | 8-12 | 5-8 |
| Moisture Absorption (%) | 9-12 | 2-4 |
| Mold Shrinkage (%) | 1.0-1.5 | 1.5-2.0 |
| Continuous Service Temperature (ºC) | 200 | 210 |
Softening Point
The significant difference in the softening points of nylon 6 and nylon 66 can be attributed to their distinct chemical structures and molecular interactions.
Chemical Structure:
Nylon 6: This polymer is made from a single monomer, caprolactam, through a ring-opening polymerization process. The repeating unit in nylon 6 is -[NH-(CH2)5-CO]-.
Nylon 66: This polymer is synthesized from two different monomers, hexamethylenediamine and adipic acid, through a condensation polymerization process. The repeating unit in nylon 66 is -[NH-(CH2)6-NH-CO-(CH2)4-CO]-.
Hydrogen Bonding:
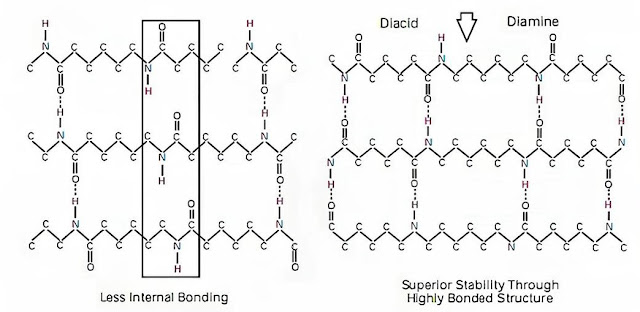
Nylon 66 has a higher density of hydrogen bonds compared to nylon 6 due to the presence of more amide groups per unit length of the polymer chain. These hydrogen bonds contribute significantly to the thermal stability and higher melting point of nylon 66.
Image shows the hydrogen bonding pattern in nylon 6 (left) and nylon 66 (right)
Crystallinity:
Nylon 66 generally exhibits higher crystallinity compared to nylon 6. The higher crystallinity of nylon 66 is due to its more regular and symmetric molecular structure, which allows for tighter packing of the polymer chains. This increased crystallinity enhances the melting point of nylon 66.
Chain Rigidity:
The structure of nylon 66 results in a stiffer polymer chain due to the presence of two amide groups per repeat unit, compared to one in nylon 6. This rigidity in the polymer chains of nylon 66 contributes to its higher melting point.
Intermolecular Forces:
The intermolecular forces, including van der Waals forces and hydrogen bonding, are stronger in nylon 66 than in nylon 6. This is due to the more extended and less flexible chains in nylon 66, which promote stronger interchain interactions.
Abrasion Resistance
Nylon 66 boasts significantly higher abrasion resistance than Nylon 6, lasting 33% longer (60,000 vs. 40,000 cycles). This advantage stems from its denser molecular structure. Higher crystallinity in Nylon 66 creates rigid blocks that hinder wear, while a more extensive network of hydrogen bonds acts like interchain tethers, resisting abrasion forces. Additionally, tighter molecular packing in Nylon 66 minimizes entry points for abrasive particles, further enhancing its wear resistance.
Low Creep and Rigidity:
The higher crystallinity in Nylon 66 also contributes to its lower creep resistance. Creep is the tendency of a material to deform under constant stress over time. Crystalline regions act as crosslinking points, hindering the deformation of polymer chains under load, resulting in lower creep.
However, this higher crystallinity also translates to slightly increased rigidity compared to Nylon 6. While this may be a disadvantage in applications requiring flexibility, the enhanced rigidity complements the superior abrasion resistance, making Nylon 66 a suitable choice for wear-prone components.
Stretch Recovery:
Despite its slightly higher rigidity, Nylon 66 exhibits good stretch recovery. This property is related to the presence of amorphous regions within the polymer structure. These amorphous regions allow for some degree of chain movement when the material is stretched. Upon release of stress, the hydrogen bonds and other intermolecular forces pull the chains back to their original orientation, enabling good stretch recovery.